Do you hope to find 'how to write nuclear decay equations'? You will find all the information on this section.
The rate of decline equation: dN/dt = -λNdN is A very small alteration in the bi of undecayed nucleidt is a same small change stylish timeso dN/dt is the rate of change of undecayed nucleithe minus communicative shows us that the number of undecayed nuclei decreases with timeλ (lambda) is called the decay constant - it's the casual any nucleus has of decaying all second
Table of contents
- How to write nuclear decay equations in 2021
- How to write nuclear equations for beta decay
- Nuclear decay equations worksheet answers
- How to write alpha decay equations
- Alpha decay symbol
- How to calculate radioactive decay
- How to do nuclear decay
- Alpha, beta gamma decay equations
How to write nuclear decay equations in 2021
 This picture representes how to write nuclear decay equations.
This picture representes how to write nuclear decay equations.
How to write nuclear equations for beta decay
 This image illustrates How to write nuclear equations for beta decay.
This image illustrates How to write nuclear equations for beta decay.
Nuclear decay equations worksheet answers
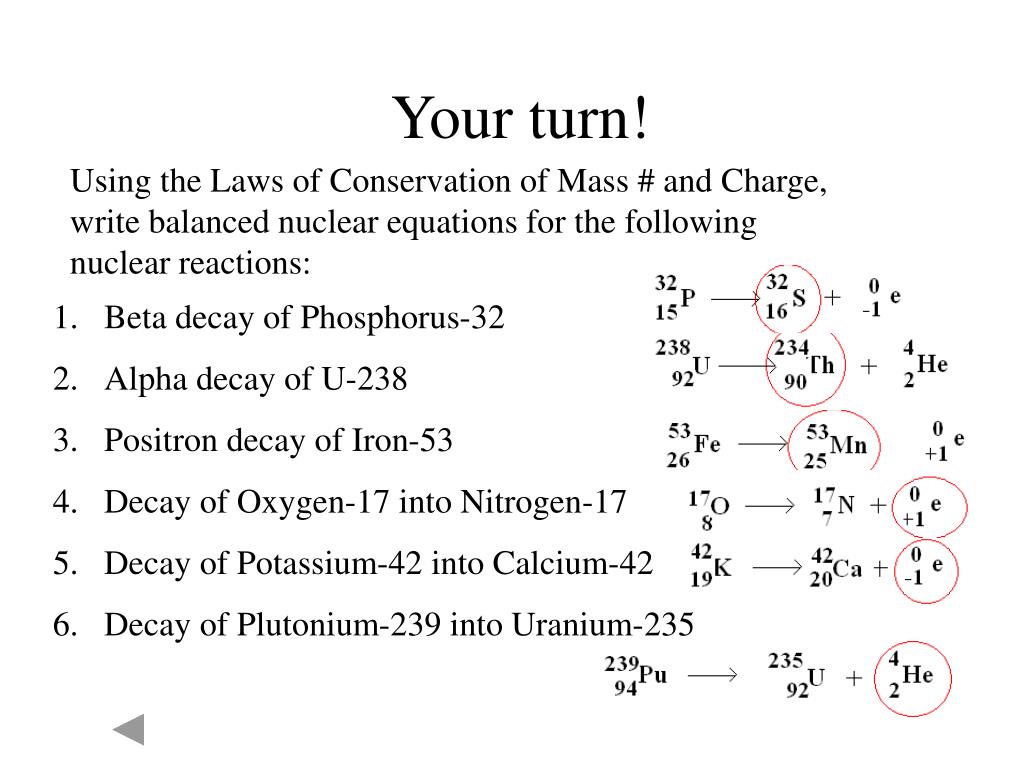 This image shows Nuclear decay equations worksheet answers.
This image shows Nuclear decay equations worksheet answers.
How to write alpha decay equations
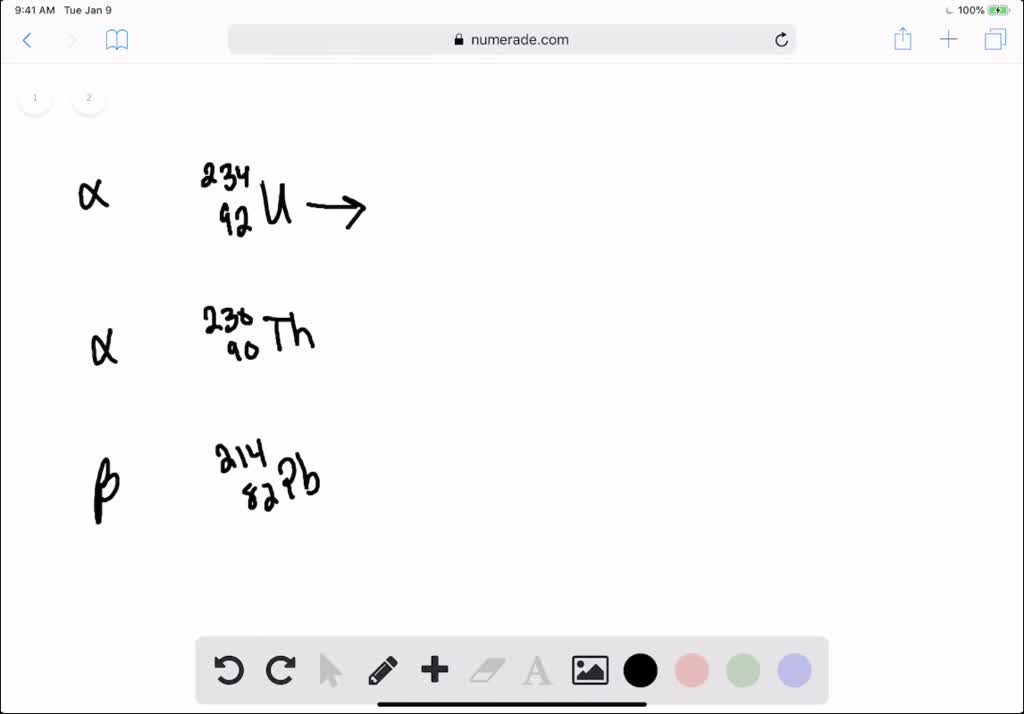 This picture shows How to write alpha decay equations.
This picture shows How to write alpha decay equations.
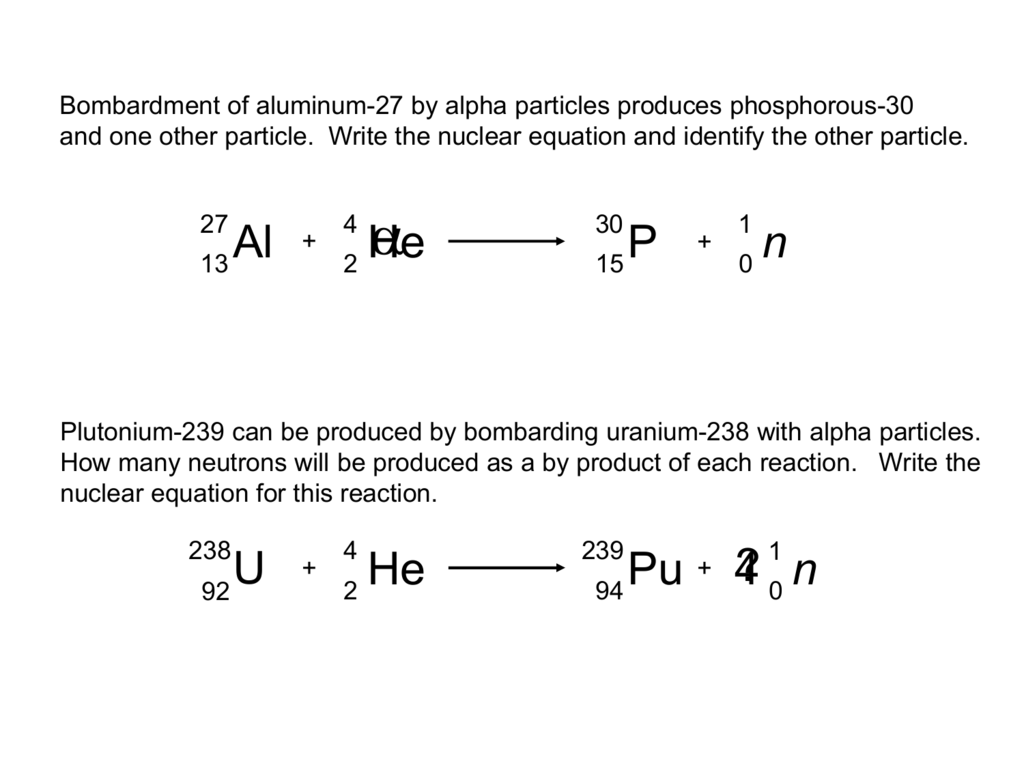
Alpha decay symbol
 This image shows Alpha decay symbol.
This image shows Alpha decay symbol.
How to calculate radioactive decay
 This image shows How to calculate radioactive decay.
This image shows How to calculate radioactive decay.
How to do nuclear decay
 This picture shows How to do nuclear decay.
This picture shows How to do nuclear decay.
Alpha, beta gamma decay equations
 This image shows Alpha, beta gamma decay equations.
This image shows Alpha, beta gamma decay equations.
How are atomic numbers calculated in beta decay?
The total of the mass numbers on the right hand side of the equation = mass numbers on left hand side of equation: The total of the atomic numbers on the right hand side of the equation = atomic numbers on left hand side of equation: The total number of nucleons has been conserved during beta decay.
Are there rules for writing out nuclear equations?
Rules for writing out nuclear equations with examples and solutions. If playback doesn't begin shortly, try restarting your device. Videos you watch may be added to the TV's watch history and influence TV recommendations. To avoid this, cancel and sign in to YouTube on your computer. An error occurred while retrieving sharing information.
How is alpha decay described in nuclear equations?
Nuclear equations A nucleus changes into a new element by emitting alpha or beta particles. These changes are described using nuclear equations. Alpha decay (two protons and two neutrons) changes the mass number of the element by -4 and the atomic number by -2.
What is the equation for the nuclear decay of radium?
Now we can complete our equation for the nuclear decay of radium-226 to produce radon-222: Note that if we add together the mass numbers on the left hand side of the equation we get the same value as for the mass number on the right hand side of the equation: A(radium) = A(alpha particle) + A(radon) 226 = 4 + 222.
Last Update: Oct 2021
Leave a reply
Comments
Gianfranco
20.10.2021 08:11Indite a nuclear par for the exploratory decay of 149 sm 62.